Research conducted by scientists at Sanford Burnham Prebys and their collaborators in Hong Kong has revealed new insights into how the three-dimensional structure of the genome impacts gene regulation. Published in the journal Genome Biology on June 27, 2025, this study emphasizes that the conventional one-dimensional view of DNA is an oversimplification that neglects the complex interactions occurring within the genome’s three-dimensional framework.
The human genome, which consists of approximately six feet of DNA, is tightly coiled around proteins called histones to fit within the nucleus of a cell. This coiled structure, known as chromatin, forms various loops and clumps. While this arrangement may seem chaotic, it plays a crucial role in bringing specific genomic regions into proximity while isolating others. Disruptions in this 3D structure have been linked to multiple diseases, including developmental disorders and various cancers. For instance, nearly 12% of genomic regions in breast cancer cells exhibit structural abnormalities, and similar issues contribute to conditions like T-cell acute lymphoblastic leukemia.
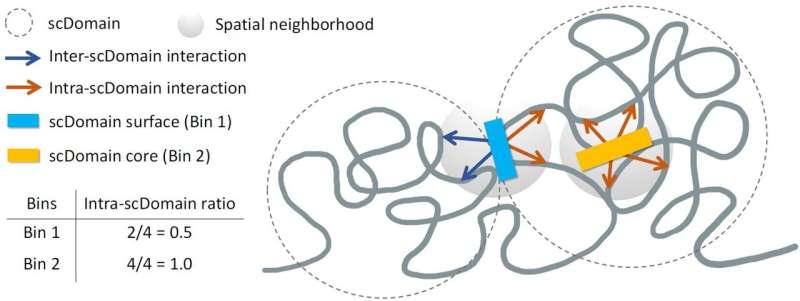
Kelly Yichen Li, Ph.D., a postdoctoral associate at Sanford Burnham Prebys and lead author of the study, stated, “We know that many regions of the genome tend to form what are known as topologically associating domains or TADs.” These domains allow parts of the genome within them to interact more frequently, while regions outside these domains remain largely isolated.
The researchers utilized advanced imaging techniques to observe the chromatin in individual cells, discovering that TAD-like regions often adopt a globular shape. According to Yuk-Lap (Kevin) Yip, Ph.D., a professor and interim director of the Center for Data Sciences at Sanford Burnham Prebys, the shapes of these regions suggest potential influences on gene function. “If you picture these clumps of chromatin fiber being roughly in the shape of a potato, we predicted that regions of the genome closer to the surface are more active due to exposure to nearby biochemical signals in the cell nucleus,” Yip explained.
The study involved the development of a new metric to assess the “coreness” of genomic regions within chromatin domains. Li elaborated, “This measure allowed us to define the surface and core, and we went on to show that surface regions are more active than core regions.” The findings present a significant opportunity for future research, as Yip noted, “The type of data we can apply this measure to is becoming quite plentiful. There is a lot of potential to study how coreness links to gene activity and disease in different cell types.”
Looking ahead, Li and Yip plan to collaborate further with the lab of Pier Lorenzo Puri, MD, to enhance understanding of how the 3D genome structure affects muscle stem cell development and the progression of muscular dystrophy. This ongoing research aims to shed light on the intersection of genetics and disease, potentially leading to new therapeutic strategies.
The implications of these findings extend beyond the laboratory, as they could inform future approaches to diagnosing and treating diseases linked to chromatin structure abnormalities. As scientists continue to unravel the complexities of the genome, the role of its three-dimensional architecture in gene regulation remains a promising area of exploration.